
Marta Barska MsC
Freelance Clinical Research Professional with 12 years experience in Drug Development from phase II up to late phase post registration studies. Since the beginning of my independent career I served to my clients as CRA, Lead CRA, Regional Project Manager, Quality Specialist and auditor.
Career progress
| 2010 - present | Independent Clinical Research professional. Served as start-up specialist, Senior CRA, Lead CRA, Regional PM, GCP trainer and GCP auditor. |
| 2007 – 2010 | Senior CRA fully outsourced to pharmaceutical company. Responsible for all aspects of studies assigned. Prepared site for audit and then FDA inspection which resulted with no findings. |
| 2006 – 2007 | Senior CRA fully accountable for all levels of clinical trials – start-up, activation, closure. Awarded as best CRA in the world for Asthma study, CRO. |
| 2005 - 2006 | CRA responsible for monitoring, site management, query resolution and closure of sites, GCP traniner and auditor, CRO. |
| 2003 – 2005 | Safety and Quality Standards Specialist, Pharma. |
Therapeutic experience
- Oncology (breast cancer, lung cancer, colon cancer, glioblastoma)
- Rheumatology (rheumathoid arthritis, psoriatic arthritis)
- Gastroenterology – ulcerative colitis, crohn disease
- Urology – BPH, overactive bladder, neurogenic bladder
- Respiratory – COPD, Asthma
- Cardiology – Anemia in Heart Failure, valve replacement, atrial fibrillation
- Orphan diseases – Fabry
- Immunology – grass pollen allergy, house mite dust allergy
- Neurology – pediatric spasticity
- Nephrology – anemia in renal insufficiency
- Orthopedics – cartilage implantation
- Medical devices – knee replacement, ulcerative colitis
- Metabolic – diabetes melitius
Services
- Monitoring Services in Poland:
- Feasibility and site selection
- information about possibilities of running particular study in Poland
- pre-feasibility for country (competitive studies, patient population, budget expectations, availability of sites and staff)
- site selection either via basic feasibility questionnaire or via online tools
- pre-study visits
- prognosis of costs
- Study start-up
- Preparation of study documents (ICF, Data protection form, contract template)
- Contract negotiations – including advisory role based on experience
- Organization of study supplies, comparators, equipment rental
- Management of patients’ travel costs
- Essential documents collection and review
- Site initiation and training visits
- Study monitoring in active phase
- Site management – regular daily contact with sites to assure appropriate conduct of study assigned
- Monitoring visits
- Appropriate preparation
- Site re-training
- Investigational product inventory and return
- Source data verification
- Investigator site file reconciliation
- Writing reports
- Follow-up on actions
- Site personnel training according to needs
- TMF management
- Pharmacovigilance follow-up
- IMP management (control of storage, shipments, destructions, return and temperature escursions)
- Ongoing communication with Regulatory bodies
- Ongoing query resolution
- Study closure
- Final query resolution
- Final TMF reconciliation
- Collection of documents from sites
- Site closure including archive
- Feasibility and site selection
- GCP Auditing Services:
- Discussion in depth with client regarding audit purpose and needs
- Preparation of audit plan in agreement with clients
- Conducting audit (onsite, TMF, CRO)
- Drafting audit report which contains:
- General overview of the audited unit
- Description of findings with ratings and risk assessments
- Suggestions of corrective actions
- Finalization of audit report
- Review of audit responses
- Advisory role in preparation of CAPA
- If contracted – final check of completion of CAPA
- Regulatory services:
- Advisory in preparation of documents needed for initial submission to Regulatory Authorities and Central Ethics in Poland
- Preparation of EudraCT form based on information provided by Clients
- Execution of site contracts mandatory for regulatory submission
- Preparation of initial study dossier needed for Regulatory bodies in Poland
- Submission of documents to CEC and Regulatory and liasing with them until approval
- Preparation and submission of Annual progress report
- Continuous communication with Regulatory Authorities and CEC throughout the study.
- Can also assist in regulatory submission for Czech Republic, Hungary, Georgia, Serbia, Russia and Latvia.
- Training services:
- GCP trainings for investigators, Site Staff, Monitors and other individuals depending on needs
- Range of training, its length and aspects are the matter of agreement between My Clients and myself
Languages:
- Polish – mother tongue
- English – proficient in speaking reading and writing
- Russian – basic in speaking, advanced in reading and understanding
Education
2001 – Medical University in Bydgoszcz MsC, Pharmacy Department, Speciality – Medical Analytics
Location
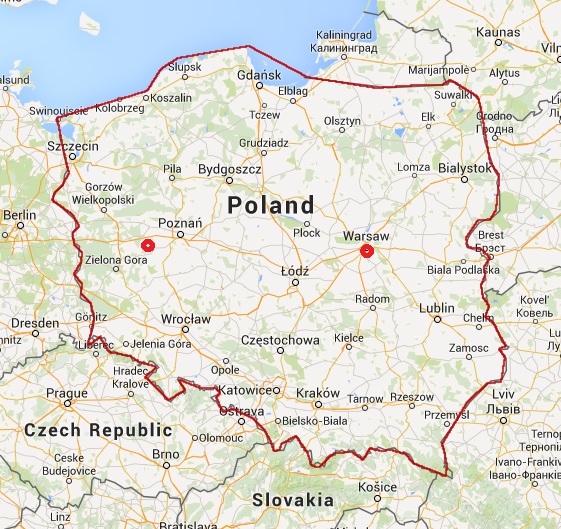
I am located in Warsaw area. I also have office near Poznań (western Poland).
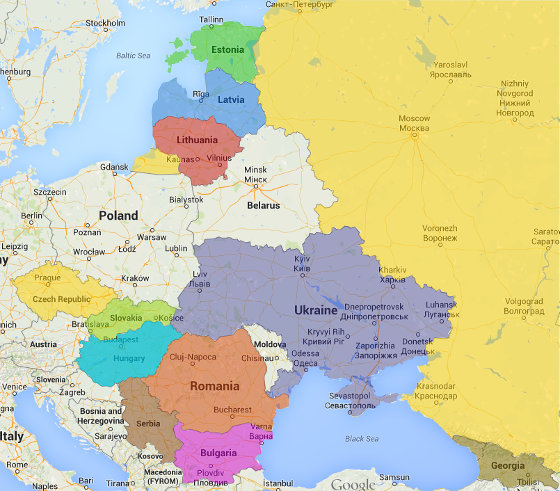
All other services are conducted in Czech Republic, Slovakia, Hungary, Serbia, Russia, Ukraine, Georgia, Lithuania, Estonia, Latvia, Romania and Bulgaria.
Contact Information
You can reach me through phone number: 0048 515 280 927
Alternatively, you can contact me by filling out the following form
